U.S. approves first twice-yearly HIV prevention shot with near-perfect efficacy
The FDA has just approved a drug that will significantly aid in the prevention and treatment of HIV-infected patients.

Yesterday, the Food and Drug Administration (FDA) approved a new, highly effective drug to prevent HIV, according to biopharmaceutical company Gilead Sciences.
This new drug, known as lenacapavir, has already passed preclinical trials and has been shown to eliminate nearly 100% of the spread of the virus when injected twice a year. Furthermore, its launch on the pharmaceutical market under the brand name Yeztugo has been confirmed. Therefore, this drug represents a light at the end of the tunnel toward the definitive eradication of the HIV epidemic.
FDA approves twice-yearly HIV shot praised as 2024’s top medical breakthrough
The FDA has approved its use in patients with HIV with just two injections per year. Furthermore, it not only eradicates 100% of the disease, but could also prevent infections in adolescents and adults weighing 35 kilos or more.
It's worth noting that the magazine "Science" highlighted this once experimental drug as the breakthrough in medicine in 2024, emphasizing that it is a "historic milestone," according to Gilead CEO Daniel O'Day. Winnie Byanyuma, former UN Undersecretary of State, also commented on the matter: "I congratulate Gilead and its US partners for promoting this important innovation," Byanyuma stated.
High cost and insurance barriers could limit access to new HIV shot Yeztugo
The potential cost of each Yeztugo injection would be approximately US$14,109 ($2,352 per month). This could be an obstacle for those lacking the necessary financial support to access this drug.
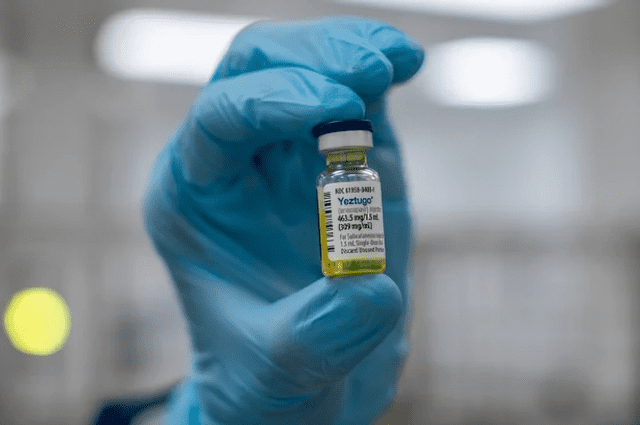
A nurse displays a dose of Yeztugo, the recently approved twice-yearly HIV vaccine considered a major breakthrough in the fight against HIV. Photo: Gilead Sciences
Furthermore, insurance companies represent another major challenge for accessing this drug, as, at least in the short term, there are other much more generic and cheaper versions, such as the drug "Truvada," which costs around US$30. On the other hand, applications to purchase Yeztugo could take up to two months to arrive because full approval would be required before it could be used.