COVID-19 vaccine update: Vaccines at risk for fall due to new FDA rules
A change in regulatory requirements could delay the availability of vaccines tailored to new variants; experts warn that they would not be ready in time if they are considered "new products."
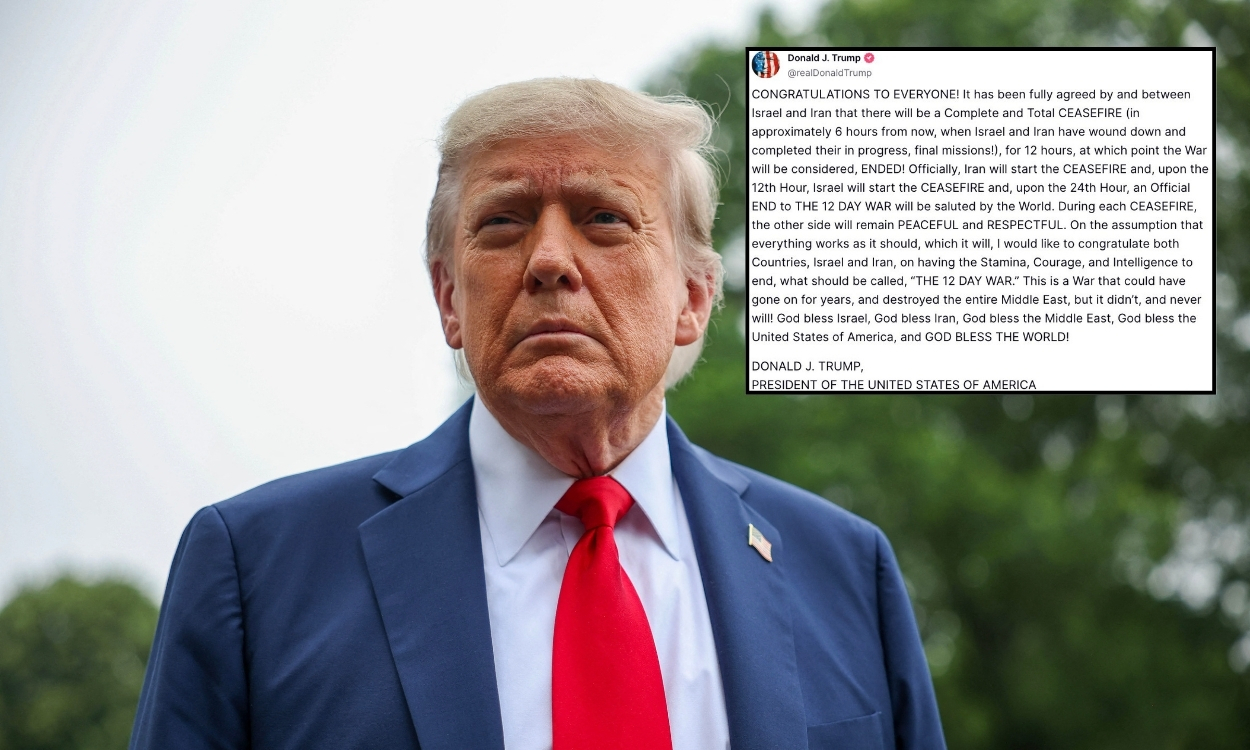
The rollout of updated COVID-19 vaccines planned for next fall could be jeopardized by a change in approval requirements issued by the U.S. Department of Health and Human Services. Under the leadership of Robert F. Kennedy Jr., certain products must undergo placebo-controlled clinical trials, a measure that could impact development timelines.
This change would have direct implications for vaccines like those from Pfizer and Moderna, which are periodically adapted to respond to emerging virus variants. If these modified versions are considered entirely new vaccines, the companies will have to conduct more extensive clinical trials, which would delay their distribution during the fall season.
New clinical trials could extend delivery times
The updated vaccines, like those for influenza, have been designed to be modified quickly based on circulating variants, without undergoing full trials each year. However, the new regulation establishes that significant changes in composition, such as the inclusion of new strains, could require additional testing for approval. According to Dr. Paul Offit, a member of the FDA's vaccine advisory committee, this requirement could delay the process by several months.
To make doses available in time, manufacturers need to know in advance which strain the FDA will recommend. The advisory committee is expected to meet in May or June to make this decision. However, experts warn that if the new rules are implemented, vaccine development and testing would not be completed before the fall.
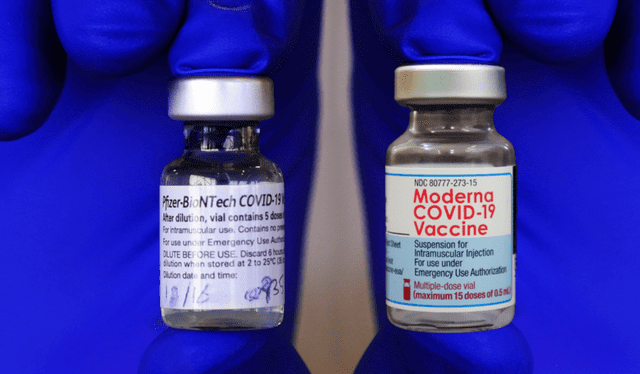
The Pfizer and Moderna vaccines are periodically adapted to respond to emerging variants of the virus. Photo: Harvard Magazine
Regulatory uncertainty affects pharmaceutical companies and vaccination schedules
Although the flu vaccine has been exempted from the new guidelines due to its history of use and proven efficacy, the same is not true for mRNA vaccines for COVID-19. Health authorities argue that the data obtained during the initial trials, more than four years ago, are no longer sufficient to authorize new formulations without updated clinical evidence.
Cases like that of Novavax, whose vaccine was delayed due to differences in the strain included compared to the original formula, exemplify the regulatory challenges companies face. If this policy continues, manufacturers could face similar delays if the stricter rules apply to their updates.