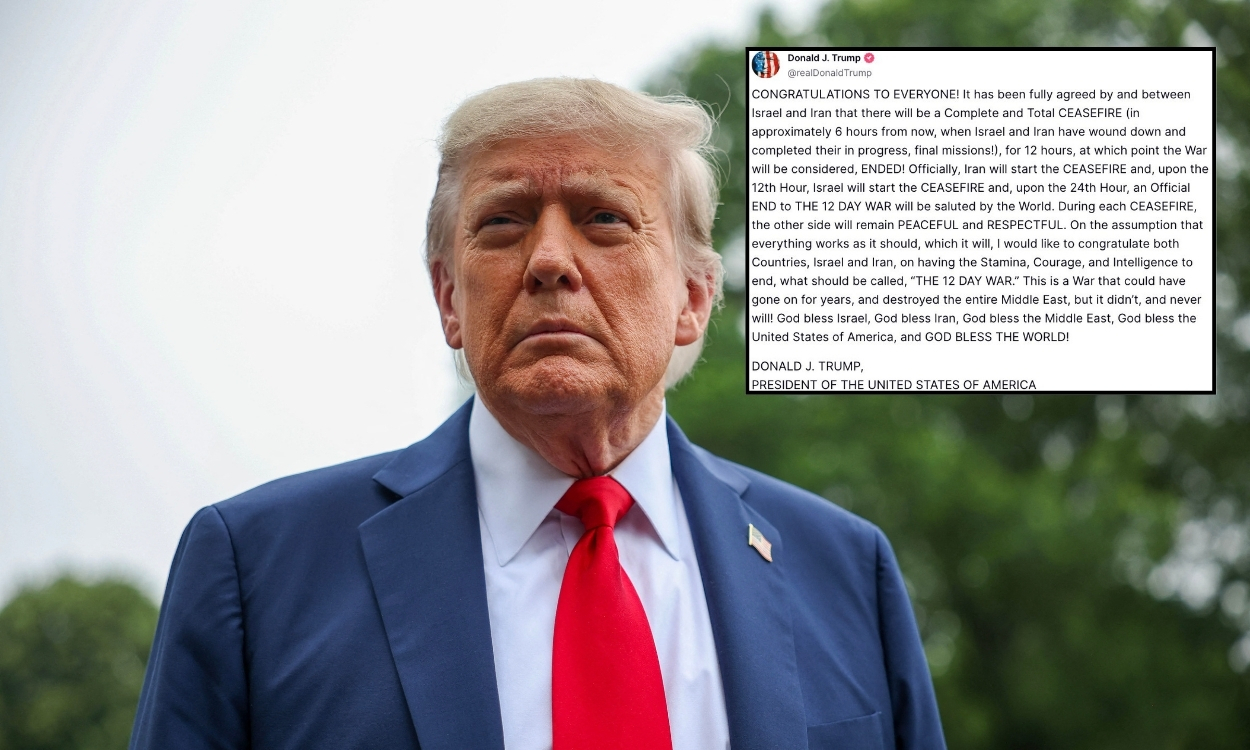
Health Warning: Nationwide voluntary recall of eye drops issued
AvKARE has issued a voluntary recall of several over-the-counter eye products following an FDA audit revealing manufacturing deviations. The recalled products, which include eye drops and gels used to treat dry and irritated eyes, may pose unknown health risks to consumers.
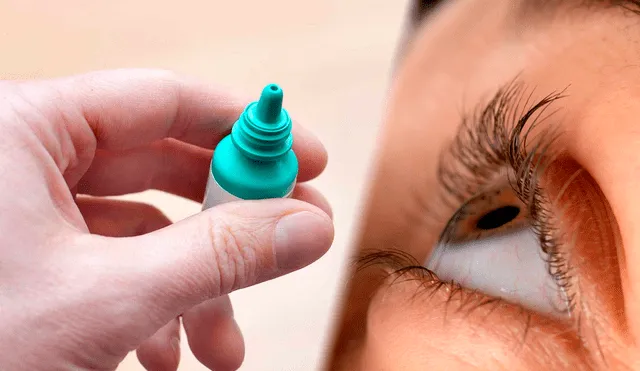
A number of non-prescription eye care items have been recalled due to manufacturing problems detailed in an FDA audit, which raised concerns regarding possible quality risks. AvKARE, a pharmaceutical distributor, voluntarily repositioned eye gels and eye drops for the treatment of dry, irritated eyes, including thousands of cases of many on the market nationwide.
While health risks may not be known, the public is advised to discontinue any eye drops and gels found in the recall. According to the company, it is offering refunds in all instances and provided on its website instructions on returning items to ensure safe disposal and reimbursement.
Which product are being recalled?
Both AvKare and the FDA have provided detailed information about the products included in the voluntary recall.
The FDA’s website lists the following affected products and quantities:
- 13,872 cases of Artificial Tears Ophthalmic Solution (National Drug Code: 50268-043-15)
- 1,610 cases of Carboxymethylcellulose Sodium Ophthalmic Gel 1% (National Drug Code: 50268-066-15)
- 32,876 cases of Carboxymethylcellulose Sodium Ophthalmic Solution (National Drug Code: 50268-068-15)
- 13,104 cases of Lubricant Eye Drops Solution (National Drug Code: 50268-126-15)
- 14,333 cases of Polyvinyl Alcohol Ophthalmic Solution (National Drug Code: 50268-678-15)
When were the products distributed?
The recalled products were shipped between May 26, 2023, and April 21, 2025. Expiration dates for the affected products range from April 2025 to March 2027.
For full details on expiration dates, lot codes, and others product information, visit the FDA's website or AvKARE's recall page.
Are refunds available for consumers?
AvKARE is advising consumers to discontinue use of the recalled products immediately.
The company is asking consumers to fill out a recall form on its website and fax the completed form to 931-292-6229 or email it to customerservice@avkare.com.
Once the form is received, the company will send consumers a Return Authorization Form to ship the product back to AvKARE. The company says it will issue full credit, including shipping cost, for any returns.